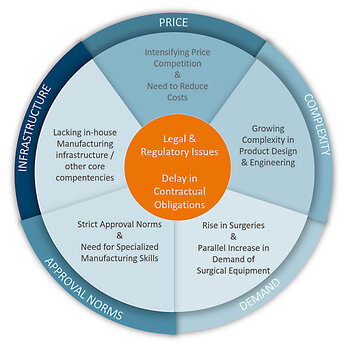
The global medical devices market offers tremendous opportunity for manufacturers, as well as significant challenges. Due to a report of the International Trade Administration’s (ITA) Global Health Team, published in 2016, aging populations worldwide, coupled with extended life expectancy, create a sustainable demand for medical devices. Presently the US-market shows the highest demands for this industry, but the European Union (EU), Japan and Canada are extremely large markets for medical devices as well. However, these stable, mature markets, have relatively low growth rates of 3 to 5 percent, as the report quotes. But analysts worldwide are sure, that the market will grow steadily. The Worldwide Medical Devices Forecast to 2020 expects a growth of total revenues from 339.5 billion USD in 2016 to 435.8 billion USD in 2020 in the leading markets like America, Asia/Pacific, Africa or Europe.
Due to a report of the EU Commission the total sales amount on medical devices in the European Union presently is about 100 billion Euro. The sector represents some 25,000 companies, of which 95% are Small and Medium-sized Enterprises (SMEs).
Outsourcing can drive the market
Medical equipment OEMs are a cornerstone of the market. But they face individual challenges. Smaller organizations often have limited development budgets, personnel resources and infrastructure. Outsourcing might help them to gain more success in competitive market segments. Larger companies on the other hand sometimes suffer from a lack of agility and need to reduce costs. Outsourcing companies can help all sizes of medical OEMs to shorten design, development and time-to-volume production. This will help to strengthen their position in a competitive field.
The medical devices sector faces many challenges at national and international level, which may have an impact on its innovation capacity and overall competitiveness. Companies need to find the balance between patient’s needs and financial sustainability. Challenges are also related to R&D, emerging technologies and the green economy. And companies need to cover issues related to international trade and regulatory cooperation globally.
Embedded computing is essential to medical devices
Players in this competitive market need to keep pace with the speed of innovation and the demands of the customers. For example, latest medical infrastructure and the need to integrate various technical items is a challenge, as is Big Data that is generated from various sources. Embedded computing platforms with high availability and high performance, that utilize the latest faster and more efficient processors are essential for this market segment as well. The design cycle of medical devices often is a multi-layered process. Development costs are high and may lead to less profitability. Outsourcing might be a solution – for start-ups and small companies, as well as for large organizations.
Partnering in the field of medical devices is a decision, that should be made carefully. The question is:
- Does the prospective partner have the technical experience a medical devices OEM needs?
- Does he offer attractive conditions?
- Does he have the capacities to deal with a project in the requested time-frame?
- Does he offer the service, that meet the specific needs of a company?
Embedded computing technology for example is a basic and very important part of any new design in the medical sector. Some medical device manufacturers do not have the competencies to develop embedded computers on their own and need external engineering expertise. Selecting Kontron as an outsourcing partner, with its wealth of design experience allows OEMs to minimize their hardware and software investments, enabling them to maximize Return-on-Investment (ROI). Kontron has a long-term experience in the embedded computing market and offers specialized medical industry expertise as well. This background helps OEMs to continually keep pace with the speed of technology. From patient care, diagnostics/imaging, radiotherapy and surgical devices, Kontron has experience in all areas of medical device development. Therefor the company can assist its partners to precisely meet their product requirements, as well as time and budgetary constraints. OEMs will get to market faster, with reduced total cost of ownership and ensured product longevity.
Quality and service are key to success
Quality is essential for medical devices and the cornerstone for success in the competitive health market. Kontron provides to its partners a controlled supply chain, that guarantees the profound controlling of a multitude of components and materials, each with specific requirements, associated standards and applicable regulations. For instance, some Kontron facilities follow the required process standards for medical devices or are ISO 13485 certified. In addition, all products are certified to UL specifications (CSA, CQC, VDE and TÜV). Another example for its high-quality standards is Kontron’s comprehensive proactive Corrective and Preventive Action (CAPA), which is applied for any complaint or non-compliance. It follows the 8D methodology for problem solving, supported by the CAPA tool, so that it can take quick action to identify, correct and eliminate recurring issues.
Quality is the key to success in the market of medical devices, as is service and support. Kontron operates global technology hubs in Europe, Asia and North America to assist its worldwide customers. Due to this service network, Kontron can offer same time-zone technical support and more personalized service. This helps medical OEMs to create innovative healthcare solutions and compete more successfully.


{{comment.comment}}